

Perfected for the use of the hot water cascading method, the Fluipharm® offers everything necessary for research and development, the production of sterile materials and, last but not least, the treatment of parenteral solutions in hospital pharmacies. After all, fast sterile reprocessing is a key ingredient to the success of any business in the pharmaceutical or biotech industry.
H model 969
1,000 x 650 x 990
644
1.918 x 1.900 x 1.360
H model 9612
1,000 x 650 x 1,340
871
1.918 x 1.900 x 1.710
H model 9618
1,000 x 650 x 1,940
1,267
1,918 x 1,900 x 2,310
Technical data subject to change without notice
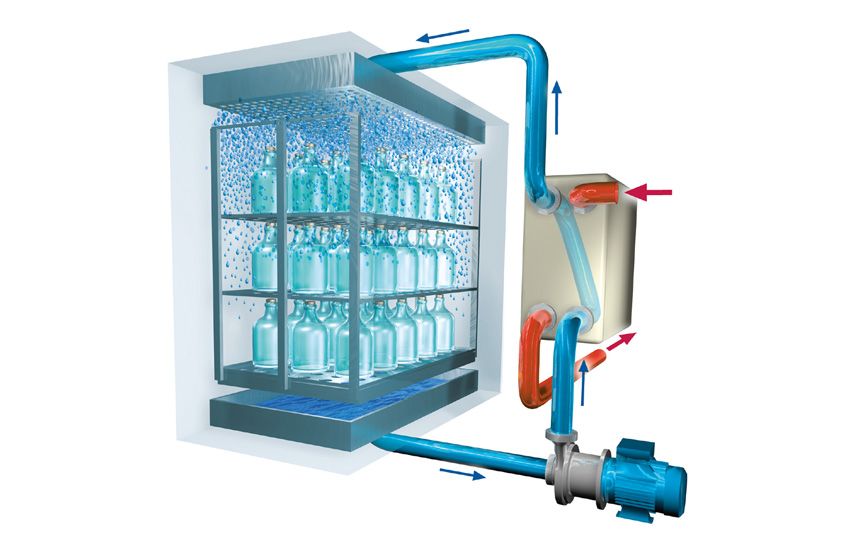
MMM developed the Fluipharm® in response to the stringent requirements of the pharmaceutical industry on design and process reliability. The machine uses the water cascading method and is ideally suited for sterilizing solid and porous materials as well as liquids in tightly sealed containers.
The Fluipharm® complies with the Pressure Equipment Directive (PED) 2014/68/EU and the Machinery Directive 2006/42/EC, CE marking according to PED 2014/68/EU. The standards applied are: DIN 58950-2 Sterilization, AD 2000 Design of Pressure Vessels and DIN EN 62304 Medical device software.
High-tech – harnessed intelligently
Combined with MMM’s SiSoft control software, the latest generation of Simatic controllers enables intuitive operation, password-protected data management, and parameter-controlled free process programmability that allows all project-specific details to be individually accounted for.
Precise process control
» State-of-the-art industrial controller
» Redundant sensors for superior process reliability
» PPV system: Process Parameter Verification
» Interfaces for optimal integration
The software
» Secure and user-friendly
» Software development and validation according to DIN EN 62304 Software life-cycle processes.
» The sophisticated parameter structure provides a high level of flexibility when configuring the machine
» User management features ensure excellent access security.
Custom machine configuration
» Continuous monitoring of all measured values
» Precise regulation of the actuators
» Barcode reading system with automatic program selection (optional)
» Autostart for automated program sequences, such as vacuum test, heating (optional)
» Versioning and release of programs (optional)
» Active P&ID diagram (optional)
» External communication interfaces, e.g., via Profinet (optional)
Conformity with 21 CFR Part 11
» User management
» Data archiving with checksum
» Audit trail
Download the brochure and find out more about the Fluipharm®
MMM Group
Semmelweisstraße 6
82152 Planegg/Munich
Germany
Costumer Service